Phytobiotics: Good morning, Kostas, I appreciate you accepted the invitation to exchange ideas about an interesting, yet challenging topic: How to measure oxidative stress and inflammation in farm animals.
The measurement of an immune defense including inflammatory and oxidative processes is not straight forward. In contrast to growth, feed intake or FCR, we cannot just apply a single metric that provides a clear numeric figure. To jump into the topic, could you explain the relation between oxidative stress and inflammation?
Kostas Mountzouris: Thank you, Tobias, for the kind invitation. Regarding your question, firstly let’s consider that the controlled generation of reactive oxygen species (ROS) and inflammatory molecules is physiological during cellular metabolism and function. For example, the healthy gut is known to be in a state of a continuous mild inflammation whereby intestinal cells counteract challenges and maintain gut homeostasis.
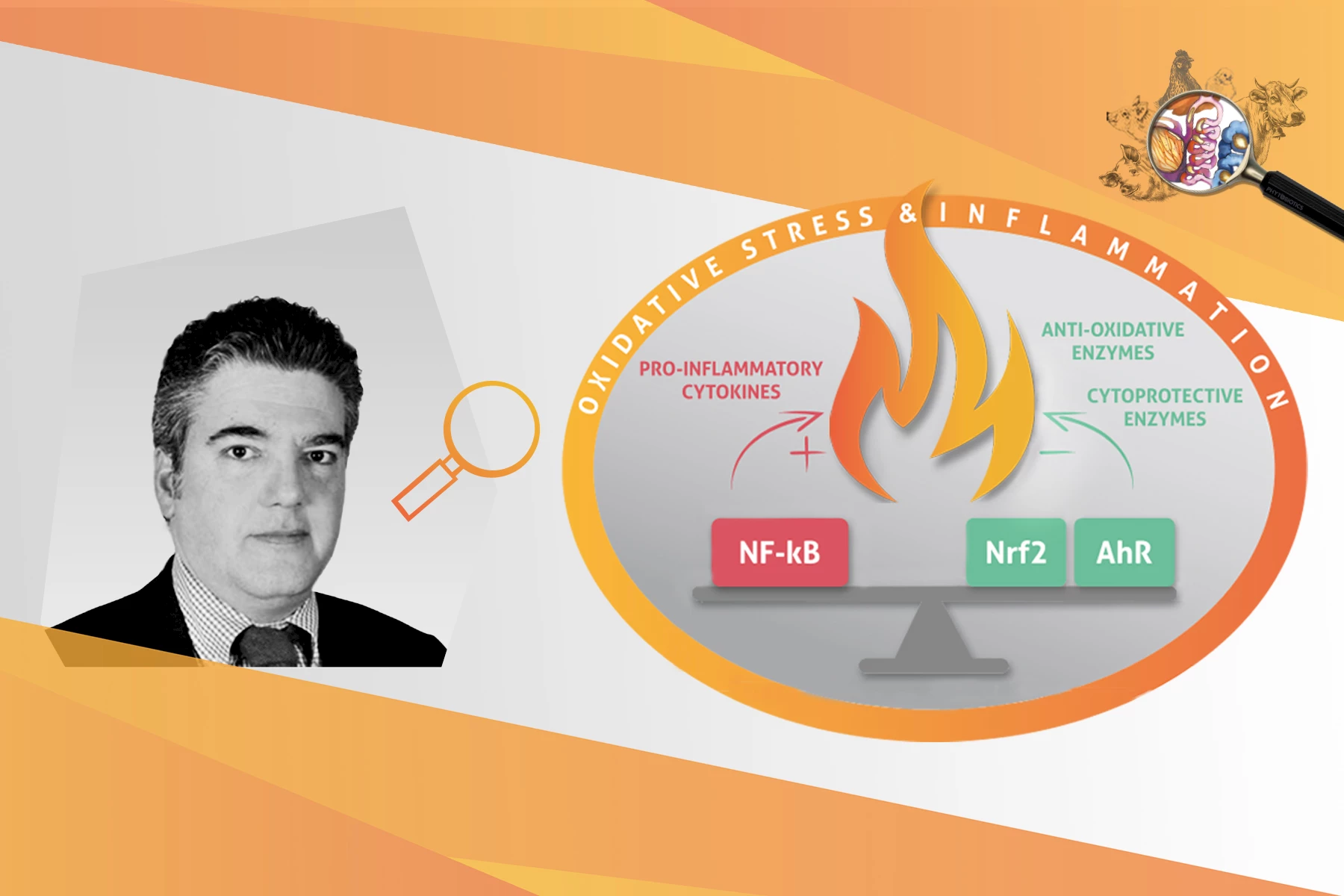
However, whenever the cellular concentration of ROS exceeds the animal’s antioxidant capacity, a dysregulation of the intracellular redox status occurs. The latter, if not promptly and adequately controlled, will lead to oxidative stress. In turn, oxidative stress will result in cellular protein oxidation and lipid peroxidation, DNA damage and activation of the transcription factor NF-κB. The latter regulates cellular immune responses to infection and higher order oxidative stress by inducing the expression of various pro-inflammatory genes. The pro-inflammatory response – if not regulated – could lead to moderate to severe inflammation, resulting in tissue damage and organ failures. Luckily, animals possess an innate detoxifying and antioxidant defense system, mastered by the transcription factor Nrf2, fit to battle oxidative stress and inflammation.
PB: Do these mechanisms occur in all parts of the body?
KM: Indeed, contemporary scientific literature provides evidence that they do occur in every part of the body such as in the liver, intestine, lungs, kidneys, and plasma.
PB: There is a saying that “all health begins in the gut”. Does your research over the last decades confi rm this, considering that oxidative/infl ammatory challenges often appear in the GIT?
KM: Yes. The gut is at the forefront line of exposure and in contact with challenge stressors of dietary and environmental origin (e.g., xenobiotics, pathogens, heat). For example, in the gut, oxidative stress negatively impacts the tight junction proteins reinforcing the intestinal barrier, opening the way for increased paracellular permeability and microbial translocation. This can lead to severe local and systemic infl ammation. It is therefore important that stressors are promptly and eff ectively counteracted at the gut level. In this respect, the stimulation and prompt activation of the endogenous cellular signaling pathways related with the innate detoxifying and antioxidant defense system are critical.
PB: What are potential mechanisms by which the animal counteracts oxidative stress?
KM: Animals may counteract oxidative stress via direct and indirect mechanisms. The direct mechanism involves the inactivation of free radicals by dietary antioxidant compounds such as vitamins, phytochemicals, trace minerals, carotenoids and cofactors such as folic acid. The indirect mechanism involves the inducible activation of two signaling pathways referred to as the AhR & Nrf2, responsible for gene expression of cytoprotective enzymes with detoxifying, antioxidant and anti-inflammatory functions. AhR stands for “aryl hydrocarbon receptor” and Nrf2 is the abbreviation for “nuclear factor-erythroid 2–related factor 2”.
PB: Delving deeper into the subject – can you explain AhR & Nrf2 in simple terms?
KM: The AhR and Nrf2 are transcription factors of the endogenous cellular signaling pathways related to the innate detoxifying and antioxidant defense system. They function as switches that turn on/off cellular protection mechanisms against oxidative stress & inflammation. For example, the AhR switch mobilizes a pathway responsible for the detoxification of xenobiotic compounds such as dioxins, mycotoxins, harmful phytochemicals and bacterial pathogens. On the other hand, the Nrf2 is a critical redox-sensitive transcription factor, known as the master regulator of cell defense against oxidation and inflammation. Essentially, when Nrf2 is activated it triggers the gene transcription of a battery of powerful antioxidant, detoxifying and anti-inflammatory enzymes that battle oxidative stress and inflammation.
PB: Are anti-oxidative and anti-inflammatory mechanisms evenly distributed across all segments of the intestinal tract?
KM: An “even” distribution of protective mechanisms and their response may not be the case in the gut. The reason is that gut motility, digestive processes and various stressors shape a dynamic environment that differs along the gut. This explains the differences seen in various biomarkers measured along the gut. Moreover, it highlights the need to understand more about the fundamental role of gut adaptive capacity for the maintenance of homeostasis. Furthermore, it is important to understand how various compounds (e.g., dietary, xenobiotics) and stressors (e.g., heat, environment) affect the adaptive ability of the gut to mount site-specific protective responses and/or signaling at systemic level (e.g., post-absorptive phase).
PB: Are there new techniques and marker genes on the horizon that may further improve our understanding of the regulation of oxidative and inflammatory processes?
KM: Yes, there are certain batteries of genes that could provide useful information on gut status and correlate with performance responses. Examples include and are not limited to genes encoding for Phase I enzymes (e.g., family of cytochrome P450 type 1 - CYP1s) as well as several Phase II antioxidant and detoxifying enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GSR), glutathione peroxidase (GPx), glutathione S-transferase (GST), NAD(P)H: quinone oxidoreductase 1 (NQO1), uridine 5-diphospho (UDP)-glucuronosyltransferase, and thioredoxin (TXN). The resulting protein are capable of preventing chronic oxidative stress, increasing toxin metabolism and preserving cellular homeostasis. I am expecting that in the near future, the mapping of the animal responses under a range of physiological and challenge stressor events will allow for the selection and promotion of the best sets of suitable biomarkers per animal species.
PB: Are there any non-invasive methods to determine the oxidative/infl ammatory status of farm animals without having to take blood or tissue samples?
KM: This is indeed a very challenging R&D topic. On the one hand, close scientifi c monitoring of zootechnical performance indices via digitalization and biosensors could inform of problematic cases. Ongoing demand for welfare and resilience indicators that correlate with health and product quality might also prove helpful. On the other hand, at a farm setting, time is critical and therefore animal health status prediction tools are clearly currently required. Here is where invasive blood analytics, like a representative herd check-up, using suitably validated biomarkers holds much promise. As a matter of fact, our group is currently interested to expand our gut platform and collaborate with other partners in this respect.
PB: Finally, what is your opinion about feed-related technologies such as diet composition or specifi c additives to modulate the oxidative/infl ammatory response?
KM: Defi nitely, nutritional interventions with carefully selected bioactive additives could provide solutions against oxidative stress and infl ammation. In this sense, nutrigenomic studies addressing the eff ects of dietary bioactive compounds, such as plant-based bioactive compounds, on the activation and magnitude of the animal’s adaptive capacity to counteract stressors leading to infl ammation are clearly required. Such studies are expected to provide the necessary tools for the mechanistic evaluation of the effi cacy of promising dietary cytoprotective technologies and applications in animal nutrition.
PB: Thank you for the interview.
Expert Kostas Mountzouris
Professor of Animal Nutritional Biotechnology, Agricultural University of Athens, Greece
Kostas Mountzouris is a Professor of Animal Nutritional Biotechnology at the Laboratory of Nutritional Physiology & Feeding of the Agricultural University of Athens (AUA) in Greece.
He holds a B.Sc. in animal science from AUA and has obtained his M.Sc. & Ph.D. in food and agricultural biotechnology from the University of Reading, UK.
His main areas of research focus on the eff ects of functional, bioactive food/ feed components and benefi cial microorganisms on physiological functions of animals such as growth performance, nutrient digestion and absorption, capacity to resist oxidation, gut microbiota composition and metabolic activity, immune response – infl ammation, gut integrity and relevant gene expressions.
Kostas has authored and co-authored 156 peer-reviewed papers and conference abstracts.
Kontaktieren Sie unsere Experten oder schicken Sie uns eine Nachricht. Wir melden uns schnellstmöglich bei Ihnen.