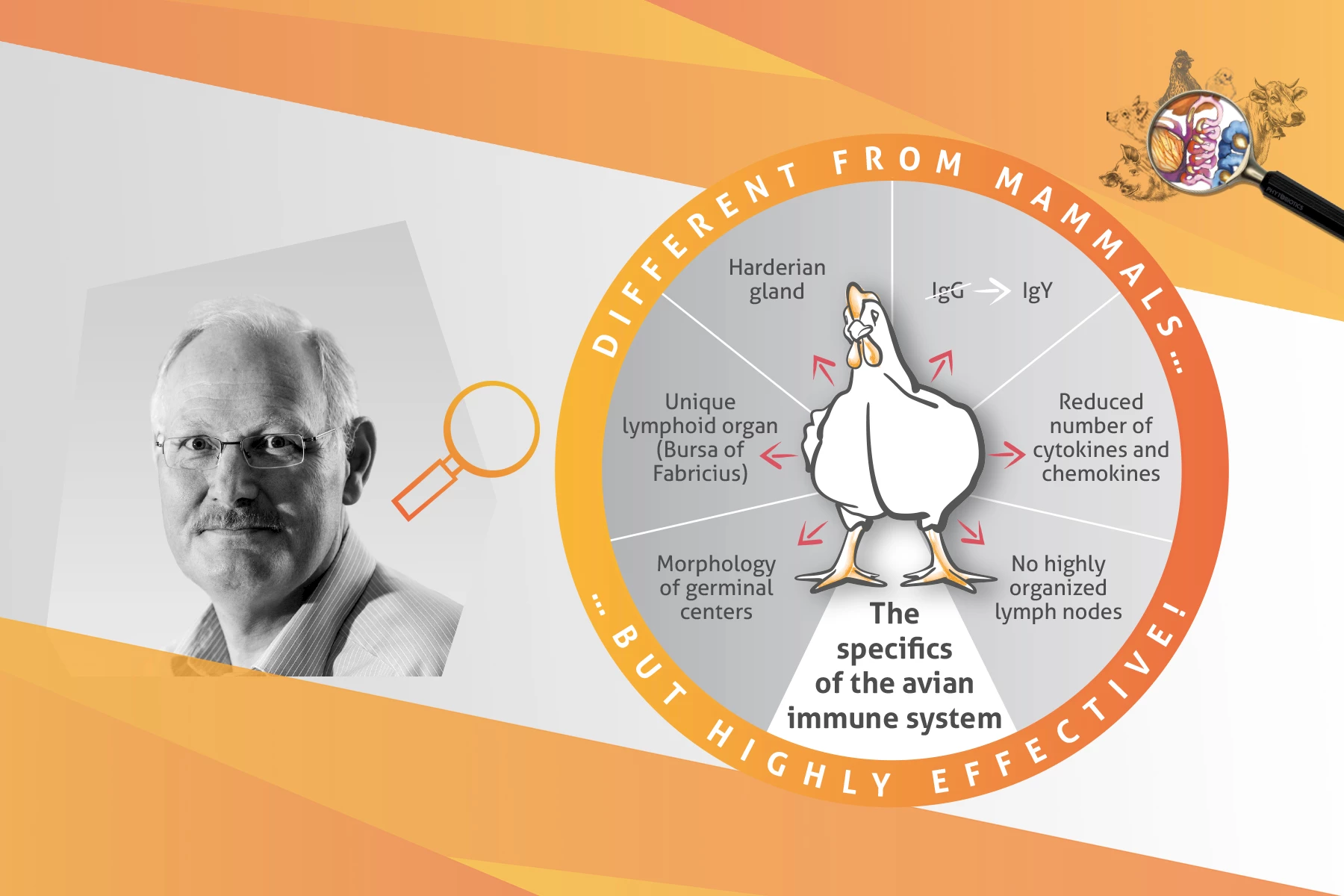
Anja Pastor: Good morning, Prof. Kaspers. Thanks a lot for being with me. Today, we would like to talk about a fascinating topic: the avian immune system. To dive right into the topic: What is the main difference between the avian immune system and the mammalian immune system?
Bernd Kaspers: Two features of the avian immune system stand out. First, birds lack highly organized lymph nodes as described in mammals. However, rudimentary lymph nodes develop in the lymphatics but do not interrupt the lymph flow. They are located in the wall of deep lymphatics and are therefore referred to as “mural lymph nodes”. Their functional relevance is largely unknown.
The second significant difference is the presence of a unique lymphoid organ, the bursa of Fabricius, only found in birds. The bursa is the site of B-cell development and its anlage appears around embryonic day 8-10 in chickens. At hatch, mature B-cells start to migrate from the bursa to secondary lymphoid tissues. Interestingly, the bursa involutes at sexual maturity. It is still not known if and where B-cells mature after bursal involution. B-cell development in the bursa has been studied intensively. In addition, numerous other structural and functional differences between birds and mammals are described, such as the small and simple major histocompatibility complex, the reduced numbers of cytokines and chemokines and the morphological difference of germinal centers. Nevertheless, birds achieve the same response to pathogenic challenges as mammals.
AP: It is interesting that birds don’t have lymph nodes. How do birds cope without them?
BK: Birds mount highly effective adaptive immune responses to infection or vaccination comparable to mammals. It is well described that germinal centers are formed in mucosa-associated lymphoid tissues (e.g., Harderian gland, bronchus-associated lymphoid tissue, caecal tonsils) and the spleen in response to immunization, leading to the formation of high-affinity antibodies and class switching from IgM to IgY (IgG) immunoglobulins. It is therefore assumed that these structures compensate for the lack of lymph nodes.
AP: In your opinion - what is the main knowledge gap in avian immunology?
BK: While the B-cell response to infection or vaccination is easy to quantify through antibody ELISA assays, specific T-cell responses are difficult to analyze. Current methods require a cell culture lab and availability of fresh organ samples. Under routine circumstances in poultry medicine, these requirements are rarely met. A major challenge is to develop test systems, which permit sampling in the field and shipment to routine laboratories for qualitative and/or quantitative analysis of T-cell responses. In addition, we need a better understanding of antigen uptake, processing and presentation in chickens in order to target vaccines and to develop new vaccination strategies.
AP: If one looks into the literature, a lot of immunological research is done in laying hens and some in broilers while there is not so much immunological research done in ducks, geese, or turkeys. Can you elaborate on the reasons for this?
BK: The community of avian immunologists is small and has largely focused on the chicken. Historically, research in chickens contributed to a number of breakthroughs in immunology. This came along with the development of specific reagents including monoclonal antibodies, recombinant cytokines and assays to quantify immune responses. Most of these reagents do not cross-react with other avian species and consequently new and species-specific tools have to be developed from scratch. Limited work has been done in ducks due to the interest in anseriformes (such as geese, ducks, and swans) as reservoirs for avian influenza viruses. Consequently, research in these species is limited.
AP: Broilers are slaughtered at quite a young age. When does a chicken have a fully developed/mature immune system? What are the consequences of this for modern broiler production?
BK: The innate immune system matures during embryonic development and is largely functional at hatch. In contrast, development of the adaptive immune system takes a few weeks after hatch.
We know that a first wave of T-cells migrates from the thymus to secondary lymphoid organs a few days before hatch but the vast majority is released after hatch. B-cells start to leave the bursa of Fabricius at hatch, but by a significant colonization of peripheral lymphoid tissues but a significant number of B-cells is not observed prior to the end of the first week after hatch.
Likewise, newly formed antibodies (initially of the IgM isotype) appear between day 3 and 7 after hatch at low concentrations followed by IgY and IgA a few days later. It is difficult to define a time when the system is fully developed but it takes at least 2-3 weeks for lymphocytes to increase in numbers in secondary lymphoid organs and mucosal tissues and up to 4 weeks to fully develop organized lymphoid structures such as germinal centers. Up to this time-point vaccination may not be fully efficient. During this period, the innate immune system and maternal antibodies provide protection from pathogenic challenge.
AP: If chicks would stay with the hen after hatching, the chicks would take up maternal microbiota. This is not possible in modern production systems. How does this have an impact on the development of the immune system?
BK: Microbial colonization of the gut is critical for the early and proper development of the immune system, both in the gut and systemically. Chickens raised under sterile conditions (germ free chickens) lack B-cells in the gut tissue and germinal center development in caecal tonsils. Consequently, plasma immunoglobulin levels are very low and no IgA antibodies are formed. Colonization with selected probiotic bacteria can induce partial maturation of the system but even combinations of up to nine bacteria do not induce full maturation if compared with maternally derived bacterial consortia provided at hatch. Future work will have to identify those strains effectively driving the development of the adaptive immune system after hatch for application in the field.
AP: Sometimes chicks have to be transported for a long distance from the hatchery to the farm. Does this kind of feed deprivation have an influence on the immune system?
BK: Immunodeficiencies are generally seen during pronounced malnutrition. Therefore, feed deprivation for a couple of hours has a minor impact on the immune system. However, stress responses associated with transport may affect animal health in several ways. Short-term stress responses lead to activation of the physiological stress axis and a higher state of immune system activation (such as increase in heterophils) which may help to control infections due to gut leakage.
AP:You and your workgroup discovered that chPTX3 – an acute-phase protein – can be used to assess inflammatory conditions in laying hens. Can chPTX3 also be used in other poultry and when should it be assessed?
BK: Acute phase proteins (APPs) are routinely used as markers of inflammation in man. They are quantified in blood samples where they may increase up to 1000-fold under such conditions. PTX3 is just one of numerous APPs and it has still to be investigated which of these proteins would be best as an early marker. A major disadvantage is the necessity to collect blood from several birds for diagnostics, which may not be practical under most circumstances. More work is required to develop good makers for inflammation and efforts are underway to identify such markers indicative of gut inflammation in feces.
We have not investigated PTX3 in other species but the high sequence homology with PTX3 between frogs and man suggests that PTX3 from other birds is highly similar to chicken PTX3.
AP: What is your Take Home Message for academia and the livestock industry?
BK: The chicken is still a fascinating model organismal for research into embryonic development and the immune system. What we need is to identify the most critical issues for the poultry industry and established joint programs, which would support the entire spectrum from basic research to application in the field, which may very well include other avian species of interest. For example, minimizing chronic stress responses and the associated immune-suppression under field conditions would be just one of these areas and would help to improve animal health and welfare.
AP: Thank you very much for the insights, Prof. Kaspers!
Expert Bernd Kaspers
Professor of Animal Physiology, LMU Munich, Germany
Bernd Kaspers is a Professor of Animal Physiology at the Faculty of Veterinary Medicine, University of Munich. He has more than 30 years of research experience in animal physiology and immunology which is documented by more than 95 peer-reviewed publications. His work involves research on both the innate and adaptive arms of the avian immune system with a particular focus on host-pathogen interaction, immune system maturation and mucosal immunity.
Professor Kaspers has developed a broad range of tools for avian immunology research such as CD-markers, recombinant cytokines, bio-assays and technologies for functional in vivo studies which have been made available for the international avian research community.
Kontaktieren Sie unsere Experten oder schicken Sie uns eine Nachricht. Wir melden uns schnellstmöglich bei Ihnen.